In my previous article, I wrote about an
introduction to material science. I believe that you understood my previous
article clearly. But I could not touch on Crystal Structures in that article.
Now I’m going to try explaining Crystal Structures in Material Science.
First, I want to define what a
crystal means. Let’s study..
If a short define is necessary, we
can say it is a repeating sequence which is appearing between solid’s atoms. In
my opinion, it is a true but not enough defining. I want to tell my model about
Crystal Structures.
Imagine a group which is consisting
of 1000 students. Probably, there are little groups in that big group that
consisted by close friends. For an
example, a class which consisted by 20 students, it means there are 50 classes
in our big group. As you know, almost in every class there are a lot of little
groups which consisted of 3, 4 or 5 students are close friends. At the outset,
we have a 1000 people group, now it is current still but we have a little
problem that is disorder due to existence of a lot of little groups. We have to
capacitate a coach who would organize them. By the way, that coach has a
whistle. The first and the only condition is students must obey that whistle. When
he blows his whistle, students must line up in a repeating sequence what their
coach wants.
Did you
hear it? Whistle is blown. Students lined up as a square after whistle was
blown. What a perfect arrangement. After coach’s gone, they disrupt their
perfect sequence and they lined up again but this time only our little
close-friends groups among of their own. So it means that, perfect crystal has
been disrupted. A new sequence existed now which is not long-term, only in
short sequences in little close-friends groups.
When an observation made from above of those
for each situation, two different shapes observe. First of them, a sequenced
perfect arrangement square when the coach is on there, second of them, that
group is in disarray, situation is distorted, when the coach releases them
free.
At the outset that big square shaped students
which we called it “crystallized” but after coach’s gone, there was a disorder.
Now, they had a short-distance sequence according to the first. And we called
that disarray “amorphous” in Material Science. It must be remembered, this
situation is not an issue. This is just a different orientation due to atom’s
natures. For an example, while metals are in crystalline forms but glass is an
amorphous solid.
Solids are
like that, too. If you can control
pressure and temperature correctly, you can obtain a crystal structure if that
material’s nature permits. Some materials can never crystallized by themselves.
You have to control environmental conditions such as pressure, temperature,
humidity etc.. If you can’t control those variables otherwise solid materials
can’t be crystallized.
Crystalline materials have a
sequence between their atoms or molecules. They can be metals, ceramics and
some polymers but except glasses. Because of glassy materials cannot be
crystallized by themselves due to they do not have long-distance sequence
according to other crystalline materials.
Types of
Crystal
Structures
Discovery of Crystallite is based
with mine and minerals sciences. In 1832, metals and their hydrate complexes
have been discovered by an English mineralogist who was W.H. Miller has found
those crystalline materials have a sequence and a selective orientation. While
Miller was investigating them, he realized that they have repeating units in a
sequence and he called those smallest repeating units were “unit cell”. He put
forth that, if those unit cells repeats their selves in a 3-dimensional
sequence, they form a solid structure. Also those unit cells have difference
characteristics each one which could be separated from the others. According to
Material Scientists, that is the most important method while identifying a
solid material as a specific fingerprint of that material.
In 1850, Auguste Bravais who has
been inspired by Miller has published his new article which was about “7
Different Crystal Structures of Solid Materials” he defined 7 different
specific lattices and combinations of their orientations at the total 14
different lattices. Also he put forth those crystal structures as a fingerprint
of minerals and solid materials which they are different from materials to
materials.
Those
different crystal structures are below.
 |
| (Source: Wikipedia) |
I can hear you’re asking “instead of might be
243 unit cells which are combinations of seven, why only existing 14 Bravais
Lattices?
I’m going
to explain this situation now why it can not be like that. In nature, only 14
Bravais Lattices exist as we know. Because face-centered, body-centered and
base-centered Bravais Lattices can not be existed in hexagonal, rhombohedra and
triclinic micro structures. If it can be like that, those structures repeat the
other base microstructures. Due to we accept the smallest lattice as a unit
cell, there are no more lattices in the nature except of 14 different Bravais
lattices.
With today’s technology, their characterization
is too easy with using XRD. X-Ray Diffractometer can determine those different
crystal structures clearly. So we can separate them which is different from the
others.
There are
amorphous solids in nature except of crystals. Glass and glassy materials do
not have any selective orientated grains. Their grains and atoms align
randomize. Those amorphous materials best example is silica which is base
materials of glasses. This is the point why glasses are transparent. Due to
they have amorphous micro-structure, their grains reflect the light randomize
so they can be seen transparent. I give their structural properties below.
 |
| Amorphous Silica- (SiO2) |
Also some special lattices are existing in nature. For an example, diamond, zinc blend, rutile etc.. I give those crystal structures at below.
I want to touch on a new material group which
are called “piezoelectric materials”and used in sensor systems. They can generate electricity when they
are below stress or vibration. How can they do that?
Their
crystal structure can yield by elastically a little at that exact moment, a
little voltage is induced and we can collect that little voltage. Their special
micro-structure is “Perovskite”.
 |
| Diamond Shape |
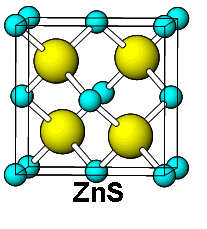 |
| Zinc Blend Structure |
 |
| Rutile (TiO2) |
 |
| Perovskite (BaTiO3) |
 |
Stress-Voltage Relationship of
Piezoelectric Materials |
I tried to put on words that, there are two
type solid materials. One of thing is “Crystallines” and the other one is
“Amorphous”. I’m thinking that my modal which I touched on “group”, it may be
benefit in terms of understanding crystalline materials. All my
studies and models belong to me. I’ll write about “Crystal defects” in my next article. Keep in
follow me please.

















